Researchers have developed a method to generate a highly specialized class of brain neurons that are central to motor neuron disease and are severely affected by spinal cord injury. The study, published in eLife, reports a successful strategy for directing rare brain progenitor cells into corticospinal-like neurons—cells that play a critical role in voluntary movement.
The editors of eLife describe the work as providing fundamental insights into the precise differentiation of adult progenitor cells into a distinct neuronal subtype. The findings establish a foundation for future studies aimed at determining whether these lab-generated neurons can integrate functionally within the nervous system and be applied to diseases involving corticospinal neuron degeneration.
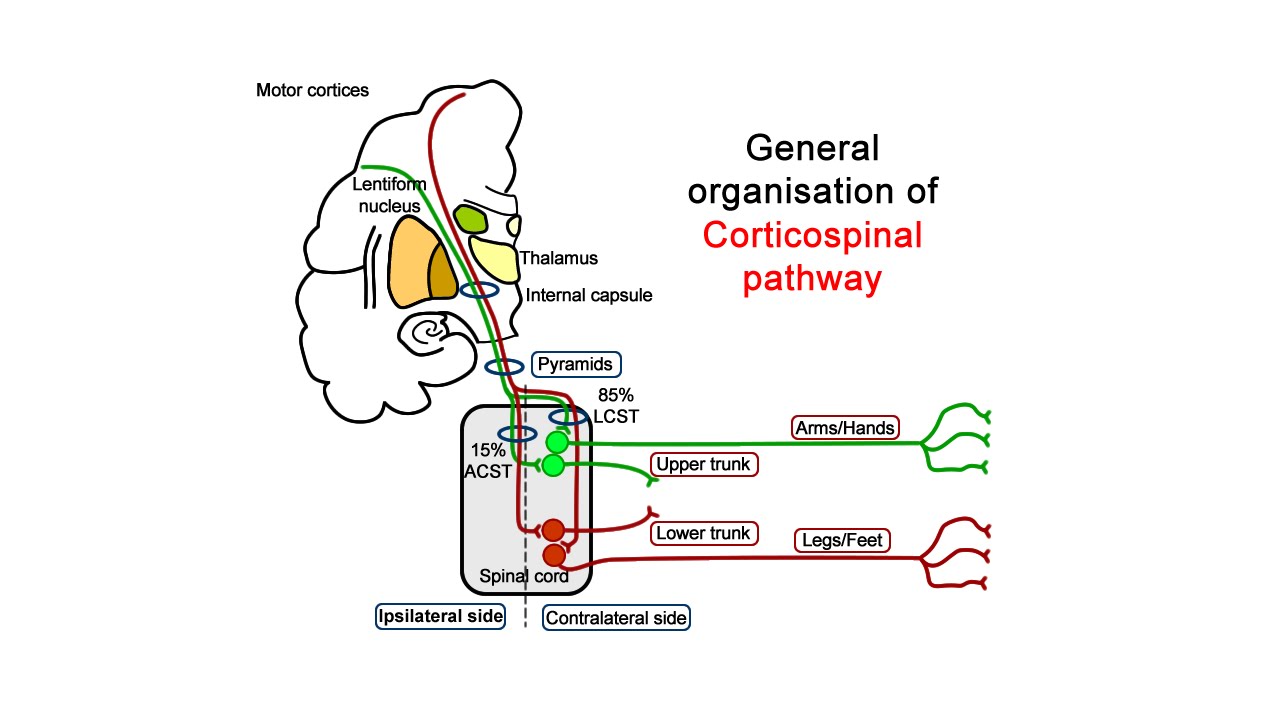
The nervous system contains a vast diversity of neurons, each defined by its structure, connectivity, gene expression, electrical properties, and function. These specialized features arise through tightly regulated developmental programs that guide immature progenitors toward specific neuronal identities. Because of this specialization, certain neuron subtypes—such as corticospinal neurons—are particularly susceptible to neurodegenerative diseases and traumatic injury.
“To accurately model disease or regenerate damaged neurons, we need precise methods to generate specific neuronal subtypes,” said co-lead author Kadir Ozkan, PhD, formerly a postdoctoral fellow in Jeffrey Macklis’ lab at Harvard University. “Generic neurons fail to reflect the selective vulnerability seen in most neurodegenerative conditions.”
Corticospinal neurons degenerate in amyotrophic lateral sclerosis (ALS) and are critically damaged in spinal cord injury, where disruption of their long axons leads to loss of voluntary motor control. Currently, there are no reliable in vitro systems that faithfully model corticospinal neuron degeneration or regeneration, significantly limiting research progress.
Building on earlier discoveries of molecular programs governing cortical neuron identity, the research team identified a population of postnatal and adult cortical progenitor cells capable of differentiating into corticospinal neurons. These SOX6+/NG2+ progenitors were found to retain latent neurogenic potential.
Using a multi-factor gene expression platform called NVOF, the researchers precisely controlled differentiation signals, producing neurons that closely matched native corticospinal neurons in morphology, molecular identity, and electrophysiological properties. In contrast, conventional differentiation methods yielded neurons with mixed identities and abnormal features.
While further studies are required to assess functional integration in living systems, the findings open new avenues for modeling ALS, studying spinal cord injury, and developing regenerative therapies.