Scientists at St. Jude Children’s Research Hospital have discovered how two major cell structures—mitochondria and lysosomes—work together to control the activation of regulatory T cells (Tregs), immune cells essential for preventing excessive immune responses. The findings, published in Science Immunology, provide new insight into how Tregs shift between active and resting states and may lead to new therapies for autoimmune diseases, inflammatory disorders, and cancer.
Tregs help resolve inflammation after the immune system fights off infections or injuries. When Tregs malfunction, inflammation can spiral out of control, potentially causing autoimmune conditions. However, the molecular processes that regulate Treg activation have remained unclear.
Using single-cell RNA sequencing in a mouse model of inflammation, researchers identified four distinct metabolic states in Tregs, ranging from a quiet resting phase to a highly activated phase before returning to inactivity. They found that Treg activation depends heavily on changes in cellular metabolism.
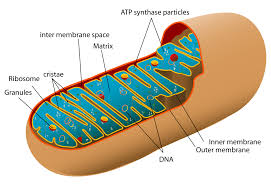
Further investigation using electron microscopy revealed increased numbers of mitochondria—with more densely folded inner membranes—in activated Tregs, suggesting enhanced energy production during activation. When researchers deleted Opa1, a gene needed for mitochondrial structure, Tregs attempted to compensate by increasing lysosome production. However, energy production and immune function still declined.
Similarly, deleting Flcn, a gene that limits lysosome activity, impaired Treg function. Both gene deletions affected TFEB, a protein that regulates lysosome-related genes, and increased AMPK signaling, a pathway that responds to low cellular energy levels. These findings show direct communication between mitochondria and lysosomes in regulating Treg activity.
Importantly, altering Flcn in Tregs improved anti-tumor immune responses in mice, shrinking tumor growth and reducing exhausted CD8+ T cells. This suggests potential for enhancing cancer immunotherapy by targeting Treg metabolism.
According to study authors, this research provides the first detailed map of how metabolism shapes Treg activation and opens new strategies for treating immune-related diseases.